The FDA Approved The First CRISPR-Based Therapy. What’s Next?
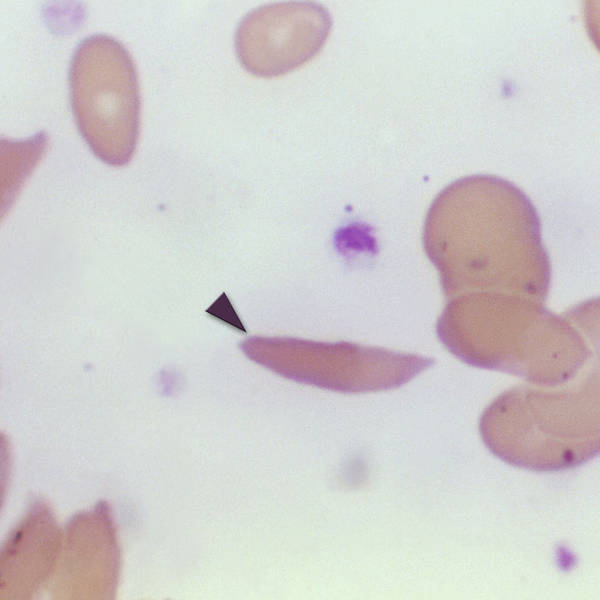
Last month the FDA approved a new treatment for sickle cell disease, the first medical therapy to use CRISPR gene editing technology. It works by identifying the gene or genes causing the disorder, modifying those genes and then returning them to the patient’s body.
There are now two gene therapies offered by pharmaceutical companies for sickle cell disease: Casgevy from Vertex Pharmaceuticals and CRISPR Therapeutics, and Lyfgenia from BlueBird Bio. But prices for these one-time treatments are steep: Casgevy costs $2.2 million per patient and Lyfgenia $3.1 million.
Both promise a full cure, which would be life-changing for patients with this debilitating condition. Over 100,000 Americans, mostly of African descent, have sickle cell disease.
This milestone raises more questions: What will be the next disease that CRISPR can help cure? And is it possible to reduce the costs of gene therapy treatments?
Ira talks with Dr. Fyodor Urnov, professor of molecular and cell biology and scientific director of technology and translation at the Innovative Genomics Institute, based at the University of California, Berkeley, about the future of CRISPR-based cures.
Transcripts for this segment will be available the week after the show airs on sciencefriday.com.
Subscribe to this podcast. Plus, to stay updated on all things science, sign up for Science Friday's newsletters.
